I copied this posting from WUS as it was just so informative. Enjoy
One of the great things about the internet is that you transmit information to millions of people all over the world; its also a major problem, as you can transmit myths and old wives tales with equal rapidity. And any attempts at killing these myths is much like playing quack-a-mole. There is much confusion about water resistance in the mind of the consumer, but from an engineering point of view, it is cut and dry.
1) The Dynamic Pressure Hydra (you chop it down here and two more posts cite it over there...)
The increase in pressure can be calculated.
The increase will be equal to the one half the density times the velocity squared, or
ΔP = (1000 x V[SUP]2[/SUP])/2
with
ΔP = increase in pressure in pascals
1000 = density of fresh water, in kg/m[SUP]3[/SUP], use 1030 kg/m[SUP]3[/SUP] if seawater,
v = velocity in m/s
(To convert pascals to atm multiply by .00001)
Doing the math, you can see that in order to raise the pressure by 1 atm the watch must be traveling at 14.25 m/s. That's about 51 km/h or 32 mph.
If you can get your arms to move that fast under water, please see your local Olympic committee, you are a due for some swimming medals this summer in London.
If you are at normal scuba diving depths (50 to 60 meters) you will have to be moving faster than a nuclear submarine to exceed the depth rating of a 100 meter rated watch. (Note: most of your military submarines do not operate deeper than 300 meters.....)
But, thats not all. Dynamic pressure only reaches its maximum at the stagnation point, which will be the point where the flow lines run into a 90 degree face. Lets look at Figure 1, below, here we have a sphere in a fluid flow, a) shows the flow lines, and b) show the pressure distribution. You can see the maximum pressure occurs only on the front face and drops off as you move around the body, and when you get to the top the pressure is actually below ambient.
So, we can see that the flow around a watch traveling through the water will see the increased pressure only on the leading surface. If the leading surface is not a gasket/case mating surface, then there is no increase in pressure on gasket.
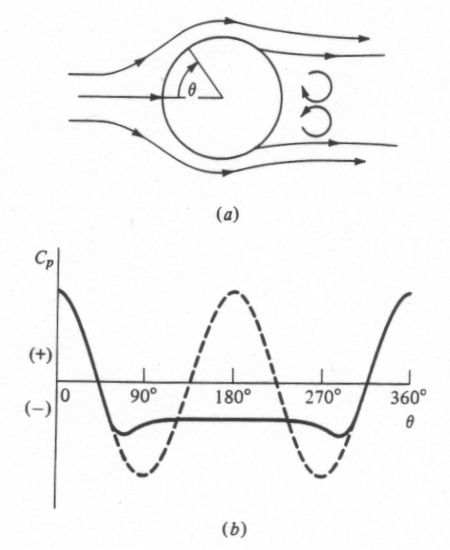
Figure 1
a) Real flow around a sphere
b) Real pressure distribution around a sphere
But wait, thats not all. Lets look at Figure 2, it is a pitot-static tube. We can see that the point at the tip of the probe is the stagnation point and the pressure here will be the total dynamic pressure. But, there is a static pressure tap on the top, the fluid must turn through 90 degrees to enter that tap, and because it does so, it does not have any component due to velocity and the pressure is the static ambient pressure. (It is ambient because the flow lines have returned to be parallel to the original flow lines, in Figure 1 the flow lines remain curved.)
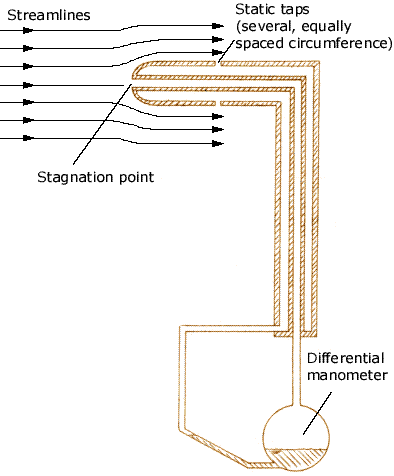
Figure 2 Pitot-static tube
So, if our watch is still traveling through the water, and the water must turn through some angle to enter a gap or crevasse, it will lose its dynamic component of pressure. All watch cases I have ever seen have the gaskets tucked behind several right angles.
So, the actual pressure increase on the gaskets of a watch being towed at 50 kph at 300 meters is not going to be the 1 atm we calculated as the dynamic pressure, but will depend on the where the gaskets are in relation to the flow lines and how many turns the fluid must make to get to them.
2) The Hot Tubs, and other hot places.
a. Heat hurting things:
Oils - The major US watch oil producer, Nye Lubricants, states the service temperature range for their oils is from -40 to 212 deg F (-40 to 100 deg C), Mobius and other Swiss oils are just as good. The oils will lose some ability to lubricate, but not enough that will affect performance of the oil.
Elastomeric gaskets (ie rubber o-rings) - Silicon rubber will maintain it elastic properties up to 450 deg F (232 deg C), Fluorocarbon Rubber (Viton) is good to 390 deg F (200 deg C). High temperatures, and by high I mean above 200 deg F, will shorten the life of elastomerics, but temperatures in the normal expected range of a worn watchwill have little or no affect on the life.
Shellac used to hold the pallet stones in place - This is the weakest point, shellac softens at 140 deg F (60 deg C) and melts at 160 to 185 deg F (70 to 85 deg C). In order for the shellac to get to 140 deg F, the interior of the case must be 140 deg F, and that means the case itself is 140 degrees or hotter.
Hairspring - Invar, Elinvar, and Nivarox are nickel-chromium steels, these material melt at temperatures in the 1450 deg F (590 deg C) range, they temper (lose springy qualities) at 650 to 700 deg F (340 to 370 deg C). Further, when they are tempered it stabilizes the material so there will be no uneven or permanent distortion when brought to high temperature (at least those below the temper range). At these low temperatures -40 to 140 deg F (-40 to 60 deg C) the only thing that these alloys will do is change volume slightly. This will change the rate of the watch ever so slightly, probably less than 0.5 sec/deg C, but the rate will return to normal as the temperature returns to room temperature.
b. Pressure Increase Due To Heat:
Gases have rather interesting thermal expansion characteristics. In 1702, Amontons discovered a linear increase of pressure with temperature for air, and found the pressure to increase about 33% from the freezing point of water to the boiling point of water. That is to say, he discovered that if a container of air were to be sealed at 0°C, at ordinary atmospheric pressure of 15 pounds per square inch, and then heated to 100° C but kept at the same volume, the air would now exert a pressure of about 20 pounds per square inch on the sides of the container. (Of course, strictly speaking, the container will also have increased in size, that would reduce the effectbut its a tiny correction, about ½% for copper, even less for steel and glass.)
Further experimentation revealed if the gas were initially at a pressure of 30 pounds per square inch at 0°C, on heating to 100°C the pressure would go to about 40 pounds per square inchso the percentage increase in pressure was the same for any initial pressure: on heating through 100°C, the pressure would always increase by about 33%. This property is true for all gases.
So, we can see that a watch assembled at room temperature (72 deg F or 22 deg C) and then heated to 212 deg F (100 deg C) would see a pressure increase of only 3.9 psi (.27 bar). No hot tub in the world (except maybe those hot tubs used by cannibals) gets anywhere near 212 deg F (100 deg C). Hot tubs may get to 110 - 115 deg F (43 - 46 deg C) at the most.
Dropping in a temperature rise of 43 deg F (22 deg C) the pressure increase will be a mere 1.125 psi (0.8 bar).
Now, given a big fashionable watch with a 30 mm crystal, the area of the crystal is 1.325 in^2 (706 mm^2), so the total force on the inside of the crystal is 1.49 pounds (6.62 newtons).
d. Not Strictly Water resistance related by related to internal pressure:
Will flying at altitude pop the crystal out? The cabin altitude of a modern commercial jet airliner is mandated to be no higher than 8,000 ft (2438 meters). That means the pressure inside the cabin will never be lower than 10.9 psi (0.75 bar). So, the maximum pressure trying to force the crystal out will be 3.7 psi (0.26 bar). However, in an effort to improve passenger comfort, few airliners have the cabin altitude that high, Boeing 767s hold 6,900 feet (2103 meters), and the Airbus 380 is quite low, at 5000 feet (1524 meters). At those altitudes the pressures are 11.34 psi (0.77 bar) and 12.25 psi (0.84 bar) respectively.
The forces on the 30 mm crystal of our watch for those altitudes are 4.32 pounds (19.2 N) for the 767 and 3.15 pounds (14 N) for the A380.
The plastic crystal in a Marathon plastic Navigator is just a friction fit and is capable of staying in place at 35,000 feet, that means it has an internal pressure 11 psi (0.75 bar) above external, thats a force of 11.25 pounds (49 N). So, once again, a well installed crystal should be able to withstand a 5 pounds (22.25 N) force trying to push the crystal from its seating.
So why do we worry about crystal popping out in saturation diving situations and not in airplanes?
Simple, the pressures involved can be much higher. Lets take a saturation habitat pressurized to 67 feet, the pressure will be twice normal atmosphere, or around 29 psi (1.99 bar). When (or if) the pressure inside the watch equalizes to the inside of the habitat it will be 2.64 times more pressure that our watch at 35,000 feet. When the watch returns to the surface, the force on the crystal is 30 pounds, which starts to approach what it takes to unseat a crystal.
3) Thermal Shock.
Watches which are tested in accordance with ISO 2281 and ISO 6425 are tested for thermal shock. A watch tested to ISO 2281, will be tested with a 36 deg F (20 deg C) thermal shock, which is going from a stabilized temperature of 104 deg F (40 deg C) to 68 deg F (20 deg C) within one minute, stabilizing the temperature again and back to 104 deg F (40 deg C), again within a minute, this is the equivalent of jumping from a chilly swim into a hot tub. For ISO 6425, the temperature jump is from 104 deg F (40 deg C) to 41 deg F (5 deg C) and back. These temperature jumps are what you would expect to see in real life.
The expansion of rubber is greater that the expansion of steel when heated, so in the heating situation the rubber will expand faster and just create a better seal.
What happens in cooling conditions? The shrinkage of rubber is greater that steel when cooled, so there is the possibility that the rubber will shrink away from the steel when cooled, opening a gap. Not likely, but possible. Why is it not likely? When you design an o-ring or gasket seal, there is a recommended compression of the gasket, given the service temperature range and type of gasket material. This is calculated so that the shrinkage of the gasket never is so great that it pulls away from the two sealing surfaces. Provided the designer paid heed to the recommendations, temperature will not affect the seal. Also, as we saw above, some changes in temperature are accounted for in testing. If it passed testing, it did not pull away enough to open a gap.
4) Soap, Hairspray and Other Chemicals...
a. Steel Corrosion
Stainless steel is called stainless steel for a reason; it is very resistant to corrosion. There are certain chemical that will eat into a stainless steel case, the most commonly encountered is seawater. Pitting, or crevice corrosion is when a corrosive substance gets into crack or small confined space and can remain stagnant. Surprisingly, the most likely place for this to occur is in and around the case back o-ring. The water seeps into the small space around the sealing area (see Figure 3, circled in red)
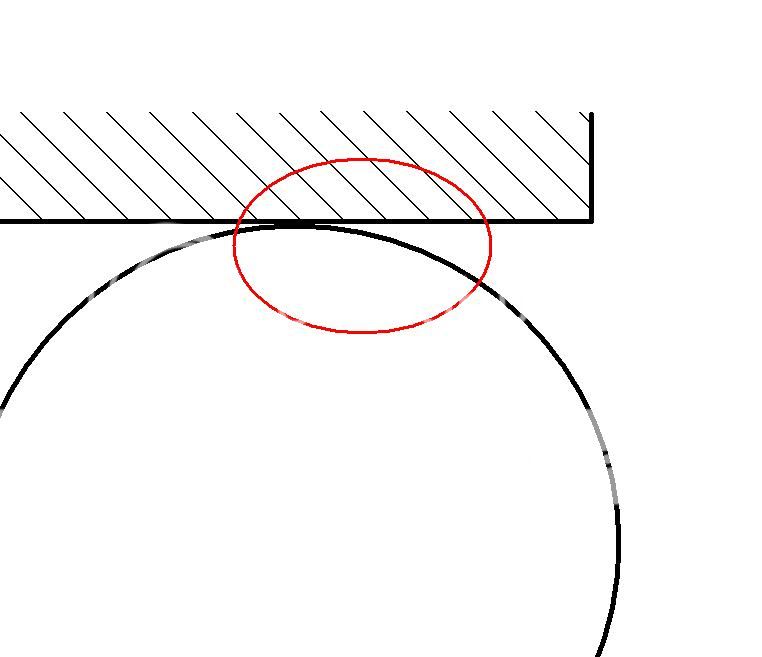
Figure 3. O-ring
The corrosion will attack the steel and eventually eat underneath the seal area. The ability of stainless steel to resist this type of attack is governed by the alloy, 904 stainless is especially good at resisting crevice and pitting corrosion, 316 and 316L are also very good (but not quite as good as 904). 304 stainless, or as it was once known 18-8 stainless, is particularly bad at resisting this type of corrosion. Seiko used this alloy extensively in the past and pitted o-ring grooves are common in used Seiko divers watches.
However, this type of pitting takes time to form, months or years, and can be prevented by simply rinsing the watch in fresh water and drying after exposure to seawater.
b. Elastometic degradation.
The elastomerics used today are either silicone or fluorosilicone, these are fairly resistant to attack from common household chemicals. In fact, they are better at resisting attack than your skin. If it doesnt hurt you, it wont hurt the gaskets. Yes, it is quite surprising that the steel case is at more risk than the rubber gaskets, but for the environment a wrist watch lives it, that's the truth.
UV will affect rubber. But, in order for UV to affect the rubber, the rubber must be exposed to UV light. How many watches do you know of with exposed gaskets? Well, the crystal gasket is exposed, isnt it? Yes, it is, but mostly from underneath the crystal. The glass in the crystal will absorb much of the UV light, reducing the damage, and the type of gasket material commonly used is nylon, which has good resistance to UV degradation. Further, UV degradation takes time, years of time, one day at the beach will not ruin the gaskets of your crystal.
Ozone will affect rubber. Thats why rubbers have a service life. The service life is anywhere from 10 to 20 years, depending on the grade of rubber and level of ozone.
5) The Truths
Sadly, this is a truth, but why? They fail because there was something wrong with the gasket, or something has damaged the sealing surfaces, in short the watch was not water resistant. It would have failed in a test chamber, under a faucet or in a hot tub, there was no special action specific to the hot tub or shower that caused failure, it was just the first time the owner noticed the problem.
What causes a water resistance failure? Well, there are those factors noted above, if it has been in service for years and the ozone or UV has broken down the rubber, or corrosion has eaten away the sealing surface. But, these actions take long periods of time to manifest themselves. More likely water resistance failure is cause by one of three things:
1) improper installation,
2) flaws, or
3) wear
New watches that fail are always improper installation or flaws; that is why you test. But, with watches tested in accordance with ISO 2281, you only need to test by sample. The sample size is not specified by ISO 2281, it is calculated by the manufacture to achieve a supportable warranty return rate. If the manufacturer decides on a rating of 3 atm, and assumes that the watch will rarely be subjected to water exposure (and given the many charts that float about stating 3 atm watches should never see a drop of water, quite likely), they may decide that one watch out of a run of 500 will be sufficient. Every single one of the other 499 watches may have pinched gaskets or cut o-rings, but the one that passed allows all the watches to proudly state tested in accordance with ISO 2281. His warranty returns may be very low (say one or two out of the entire batch) as the owners are leery of exposing a 3 atm watch to water...
Wear is the more insidious failure mode, as the rate at which it occurs is unknown and variable. In non-screw down crown, winding will continually rub the gasket against the case tube; this will abrade the rubber off and reduce the seal quality, minor variations in the surface finish change the speed at which the rubber abrades.
Screw down crowns are less subject to wear, but will see it. In old divers they sometimes used sealing just like non-screw down crowns and are subjected to abrasion in the same way as noted above. In newer divers, with a compression gasket at the top of the crown, the wear only occurs during the final stages of screwing down the crown. Wear occurs, it just take longer to become a problem.
Case backs are not subject to wear if they are static. So, unless you (or your watch guy) are in the habit of reusing gaskets you will never have a case back gasket wear problem.
Upshot of this post? If you plan on getting your watch wet, have it tested periodically, every 12 to 18 months, and you should never have a problem washing your hands, or showering, even with a 3 atm rated watch. A properly tested 10 atm rated watch should withstand anything water related...
