I wanted to take a few moments to share my thoughts on why some brushes may shed, either initially or after months to years of use. It is a common topic here on B&B and I am by no means suggesting I am right or that my thoughts are all inclusive, rather, I wanted to share some of my experiences along with some pictures which may help explain why some brushes shed.
As with almost anything else in life, the outcome of an event is often shaped by several variables, and I suspect the same is true with brushes which shed. Though some of those variables are user-dependent, i.e., user care, frequency of use, etc, some are not and that is what I hope to address in this post, at least in part.
Most knots have hairs/bristles which are set in an adhesive base, commonly referred to as a knot plug. I have used and held knots from several vendors and while the type of adhesive/epoxy used for this process may be different, there seems to be one universal truth to almost every knot I have held in my hand- there are bubbles in the adhesive base, or knot plug. While the bubbles are small, some near microscopic, they are always there and essentially represent a void in the plug. This is not a criticism of any vendor/supplier, simply an observation and an unavoidable truth.
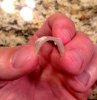
Here is a knot from a well respected vendor I and many others use. Due to the color of the adhesive, it is hard to see the bubbles in the plug.

Here is the same plug under 30x magnification with my loupe. The bubbles are readily visible now and appear innumerable.

Here is the same knot, showing the same plug from the side demonstrating macroscopic bubbles along the upper edge of the plug.

I use a similar epoxy to set my knots in place and I hope to demonstrate how these bubbles come about and how they, coupled with regular use/care, may contribute to some shedding.
Here is a freshly poured 2 part epoxy commonly used to set knots into a handle. As you can see, when both parts are dispensed, there are no bubbles present.

However, even mixing the 2 parts VERY slowly, yet thoroughly, lots of air is introduced to the mix and due to the viscosity of the substrate, remain, even when fully cured as I hope to demonstrate in the following post.

As with almost anything else in life, the outcome of an event is often shaped by several variables, and I suspect the same is true with brushes which shed. Though some of those variables are user-dependent, i.e., user care, frequency of use, etc, some are not and that is what I hope to address in this post, at least in part.
Most knots have hairs/bristles which are set in an adhesive base, commonly referred to as a knot plug. I have used and held knots from several vendors and while the type of adhesive/epoxy used for this process may be different, there seems to be one universal truth to almost every knot I have held in my hand- there are bubbles in the adhesive base, or knot plug. While the bubbles are small, some near microscopic, they are always there and essentially represent a void in the plug. This is not a criticism of any vendor/supplier, simply an observation and an unavoidable truth.
Here is a knot from a well respected vendor I and many others use. Due to the color of the adhesive, it is hard to see the bubbles in the plug.

Here is the same plug under 30x magnification with my loupe. The bubbles are readily visible now and appear innumerable.

Here is the same knot, showing the same plug from the side demonstrating macroscopic bubbles along the upper edge of the plug.

I use a similar epoxy to set my knots in place and I hope to demonstrate how these bubbles come about and how they, coupled with regular use/care, may contribute to some shedding.
Here is a freshly poured 2 part epoxy commonly used to set knots into a handle. As you can see, when both parts are dispensed, there are no bubbles present.

However, even mixing the 2 parts VERY slowly, yet thoroughly, lots of air is introduced to the mix and due to the viscosity of the substrate, remain, even when fully cured as I hope to demonstrate in the following post.